| The Chemistry of Paints and Painting is a free textbook on chemical aspects of painting. See the editorial for more information.... |

|

Home  Pigments Pigments  Yellow Pigments Yellow Pigments  Yellow Ochre Yellow Ochre |
|||||||||||||||||||






|
|||||||||||||||||||
|
Yellow Ochre
The distinction between the yellow ochres and the red ochres, whether natural or artificial, depends upon a perfectly definite chemical difference. The colour of every one of these pigments is due, indeed, to iron, and to iron in the same state of oxidation; but the iron oxide in the yellow and brown ochres is chemically united to water, while in the red ochres it is nearly or quite anhydrous - that is, dry. In chemical language, then, we may say yellow ochre is a ferric hydrate, red ochre a ferric oxide. But, when we proceed to examine a number of samples of yellow ochre, we find, not merely different proportions of ferric oxide to combined water - that is, different ferric hydrates - but we find also very variable proportions of intruding or accessory constituents. In fact, yellow ochre represents not less than three mineral species, and it occurs associated with many impurities, the latter consisting mainly of silica, of clay, of rocky debris, with traces of gypsum, of iron or copper pyrites, and of humus or peaty acids. There are, moreover, ochres in which other compounds occur, as barium sulphate to the extent of 75 per cent in some American varieties. The three fundamental minerals, in order of frequency, which maybe traced in various yellow ochres, are these: Brown hematite, or limonite, consisting of two molecules of ferric oxide combined with three molecules of water, and represented by the formula 2Fe2O3 Yellow hematite, or xanthosiderite, consisting of one molecule of ferric oxide combined with one molecule of water, and represented by the formula Fe2O3 Bog-iron ore, or lymnite, consisting of one molecule of ferric oxide and three molecules of water, and is represented by the formula Fe2O3 It is probable that all the numerous varieties of yellow ochre, from the countless localities of this substance, belong essentially to one or other of the above species of iron minerals, although the frequent presence of such impurities or accessories as silica, iron silicates, and clay renders the identification very difficult. Moreover, there are reasons for suspecting, in some ochres at least, the presence of another and more complex compound, namely, a distinct double iron-aluminium hydrate. An analysis of a fine sample of yellow ochre, taken by the author from a pit on Shotover Hill near Oxford, gave the following percentages:
The varying hues of yellow ochres depend mainly upon two differences of composition. One of these is the amount of white clay, silica, calcium sulphate, or barium sulphate present in them - this lightens the colour; the other is the presence of ferric oxide, which gives them a ruddier or warmer hue. All, when burnt - that is, calcined - lose their essential water, and become converted into various kinds of red ochre, light red, etc. The varieties which contain much silica and clay (ingredients which, even in good yellow ochres, often amount to two-thirds of their weight) yield the less translucent and paler tints of some of the burnt red ochres. India furnishes a great variety of hues of yellow ochre, but our chief supplies come from France, Italy, Germany and Spain. More recently excellent ochres have been obtained from the district of Dubbo in New South Wales. Some of the English ochres (from Oxfordshire, Derbyshire, etc.) are of fine quality. Peri-gord Yellow, a natural earth found in Perigord, is a fine variety of yellow ochre: it yields when heated to 800°-1,000° C. a fine reddish orange, brighter than that of the light red produced from any other ochre. Yellow ochre is generally prepared for use as a pigment first of all by careful selection of the best pieces, and then by the familiar process of elutriation, or washing over. Thus it is at once freed from sand or other coarse particles, and from any soluble salts which it may contain. Immediately before being ground in oil, it should, however, be dried at a temperature a little below that of boiling water, as it is liable to contain hygroscopic moisture in addition to its necessary constitutional water. Yellow ochre is one of the most ancient pigments, having been used by the Egyptians, the Greeks, and the Romans. It is the oichra of Theophrastus. Pots of yellow ochre were found at Pompeii. It has stood, with very little change, the test of centuries. It certainly does become, in all media, but especially in oil, slightly darker and warmer in hue after prolonged exposure to light. The change, however, is slight; moreover, it soon comes to a stop. It is probably due in part to a slight loss of constitutional water from the ferric hydrate, and in part to increased translucency. It must be recollected also that yellow ochre as an oil-paint contains 40 or more percent of oil, and this becomes yellower and darker in time. Yellow ochre, so long as it is exposed to air and light, is not darkened by sulphuretted hydrogen. It is without action on other pigments, although the statement has often been made, on quite insufficient grounds, that paints which are damaged by contact with metallic iron are likewise damaged by yellow ochre and by the red oxide of iron. For instance, true Naples yellow is undoubtedly spoilt by contact with a steel spatula, because the metal of the latter takes away oxygen from, or 'reduces' the lead antimoniate of which the former consists. But such an action is impossible with yellow ochre, for this iron compound is a stable substance, containing already all the oxygen it can take up. It is possible, notwithstanding, that ochre may injure the hue of some lakes, such as yellow lake and crimson lake, by replacing in part some of the alumina with which the colouring matter is united. But as such lakes are worthless, from their extreme instability when exposed to light, when used alone, such probable action of ochre upon them need scarcely be considered. Still the same action may occur in the case of the madders and alizarin pigments. Yellow ochre is little subject to adulteration, for it is too cheap a pigment to make it worth while to substitute other substances for it. But sometimes the golden and richer coloured varieties have been found to have had their colour enhanced by the addition of certain fugitive or semi-permanent yellows of artificial or organic origin. The majority of such additions may be detected by pouring a little liquor ammoniŠ mixed with spirits of wine upon some of the ochre placed on a filter-paper in a funnel: the liquid passing through will be colourless if the ochre be genuine. An ochre which when heated in a test-tube gives off, besides water, fumes which partially condense into a coloured or tarry matter on the glass, contains organic matter, naturally present or artificially added, and is generally of inferior permanence. Of late years a far more frequent adulteration of yellow ochre is the addition of chrome yellow - that is, lead chromate. This adulteration may be detected by boiling the suspected ochre with sodium carbonate solution, filtering, and adding to the nitrate enough acetic acid to neutralize it, and then a few drops of lead acetate. A yellow precipitate indicates the presence of a chromate. An artificial yellow ochre is made by acting upon solutions of iron salts with metallic zinc, and thoroughly washing the precipitate obtained. Brown ochre is an approximately pure limonite: raw sienna is very nearly related to it (see farther on), but cologne earth, raw umber, Caledonian brown and vandyke brown are distinct substances. An artificial brown ochre is prepared by heating yellow ochre with 4 percent of common salt to a low red heat. Under the name of cyprusite a peculiarly bright lemon-coloured earth has been imported from Cyprus as a pigment: it consists essentially of a hydrated ferric sulphate: it is not likely to prove a safe pigment for artistic use.
|
|||||||||||||||||||
Home  Pigments Pigments  Yellow Pigments Yellow Pigments  Yellow Ochre Yellow Ochre |
|||||||||||||||||||
Last Update: 2011-01-23


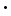 3H2O;
3H2O;