| The Chemistry of Paints and Painting is a free textbook on chemical aspects of painting. See the editorial for more information.... |

|

Home  Pigments Pigments  Red Pigments Red Pigments  Cobalt Red Cobalt Red |
|||






|
|||
|
Cobalt Red
This little-used pigment should consist of the oxides of magnesium and cobalt. It is prepared at a high temperature and is quite permanent. One method of making this pigment involves the use of magnesium carbonate or oxide, which is made into a paste with a solution of pure cobalt nitrate. This paste is then slowly dried, and ultimately calcined in a crucible. Different preparations of this pigment differ considerably in hue; a purplish cast is sometimes due to the accidental presence of alumina. Pigments consisting of cobalt arseniate are occasionally called 'cobalt red'; the term 'cobalt violet' is usually and may be more fitly applied to them. The hue they present is rather bluer (or less red) than that of the flowers of the common foxglove. Cobalt violet has been made from the mineral known as erythrite, or cobalt-bloom, which has the formula Co3As2O8
|
|||
Home  Pigments Pigments  Red Pigments Red Pigments  Cobalt Red Cobalt Red |
|||
Last Update: 2011-01-23


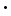 8H2O; an artificial cobalt arseniate is also made by oxidizing cobalt sulph-arsenide, which is first reduced to powder and then roasted with twice its weight of potassium carbonate. After further treatment, the final product obtained by grinding and washing constitutes a pigment of a rather coarse grain which does not work smoothly as a water-colour, but has the advantage of complete stability in all vehicles. A sample of this cobalt violet of good quality was found to contain no water and to suffer no change when heated to a red heat in the air; along with cobalt arseniate it contained some phosphate.
8H2O; an artificial cobalt arseniate is also made by oxidizing cobalt sulph-arsenide, which is first reduced to powder and then roasted with twice its weight of potassium carbonate. After further treatment, the final product obtained by grinding and washing constitutes a pigment of a rather coarse grain which does not work smoothly as a water-colour, but has the advantage of complete stability in all vehicles. A sample of this cobalt violet of good quality was found to contain no water and to suffer no change when heated to a red heat in the air; along with cobalt arseniate it contained some phosphate.