| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Physical Measurements Physical Measurements  Absolute Units Absolute Units |
||||||||||||||






|
||||||||||||||
Absolute Units
The difficulty, however, of obtaining an arbitrary standard which is sufficiently permanent to be reproducible makes this arbitrary method not always applicable. A fair example of this is in the case of measurement of electro-motive force,1 for which no generally accepted arbitrary standard has yet been found, although it has been sought for very diligently. There are also other reasons which tend to make physicists select the units for a large number of quantities with a view to simplifying many of the numerical calculations in which the quantities occur, and thus the arbitrary choice of a unit for a particular quantity is directed by a principle of selection which makes it depend upon the units already selected for the measurement of other quantities. We thus get systems of units, such that when a certain number of fundamental units are selected, the choice of the rest follows from fixed principles. Such a system is called an ' absolute' system of units, and the units themselves are often called 'absolute,' although the term does not strictly apply to the individual units. We have still to explain the principles upon which absolute systems are founded.
q = where α, β, γ may be either positive or negative, whole or fractional. The following instances will make our meaning clear:
We may thus take the relation between the numerical measures
q to be the general form of the expression of an experimental law relating to physical quantities. This may be written in the form
This equation, as we have already stated, expresses a relation between the numerical measures of the quantities involved, and hence if one of the units of measurement is changed, the numerical measure of the same actual quantity will be changed in the inverse ratio, and the value of k will be thereby changed. We may always determine the numerical value of k if we can substitute actual numbers for q, x, y, z, ... in the equation (1). For example, the gaseous laws may be expressed in words thus: 'The pressure of a given mass of gas is directly proportional to the temperature measured from -273° C, and inversely proportional to the volume,' or as a variation equation
p or p = kθ/v We may determine k for 1 gramme of a given gas, say hydrogen, from the consideration that 1 gramme of hydrogen, at a pressure of 760 mm of mercury and at 0°C, occupies 11200 cc. Substituting p=760, θ=273, ν=11200, we get k = 760 x 11200 / 273 = 31180 and hence
Here p has been expressed in terms of the length of an equivalent column of mercury; and thus, if for ν and θ we substitute in equation (2) the numerical measures of any volume and temperature respectively, we shall obtain the corresponding pressure of 1 gramme of hydrogen expressed in millimetres of mercury. This, however, is not the standard method of expressing a pressure; its standard expression is the force per unit of area. If we adopt the standard method we must substitute for/ not 760, but 76 x 13.6 x 981, this being the number of units of force2 in the weight of the above column of mercury of one square-centimetre section. We should then get for k a different value, viz.: k = 1014000 x 11200 / 273 = 41500000, so that
and now substituting any values for the temperature and volume, we have the corresponding pressure of 1 gramme of hydrogen expressed in units of force per square centimetre. Thus, in the general equation (1), the numerical value of k depends upon the units in which the related quantities are measured; or, in other words, we may assign any value we please to k by properly selecting the units in which the related quantities are measured. It should be noticed that in the equation q = k xα yβ zγ.... we only require to be able to select one of the units in order to make k what we please; thus x, y, z,... may be beyond our control, yet if we may give q any numerical value we wish, by selecting its unit, then k may be made to assume any value required. It need hardly be mentioned that it would be a very great convenience if k were made equal to unity. This can be done if we choose the proper unit in which to measure Q. Now, it very frequently happens that there is no other countervailing reason for selecting a different unit in which to measure Q, and our power of arbitrary selection of a unit for Q is thus exercised, not by selecting a particular quantity of the same kind as Q as unit, and holding to it however other quantities may be measured, but by agreeing that the choice of a unit for Q shall be determined by the previous selections of units for x, y, z,... together with the consideration that the quantity k shall be equal to unity.
|
||||||||||||||
Home  Physical Measurements Physical Measurements  Absolute Units Absolute Units |
||||||||||||||
Last Update: 2011-03-27


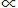 xαyβzγ....
xαyβzγ....