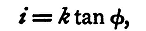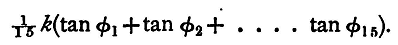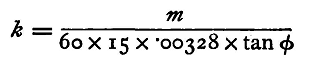| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Electricity Electricity  Determination of the Reduction Factor of a Galvanometer Determination of the Reduction Factor of a Galvanometer |
||||||






|
||||||
Determination of the Reduction Factor of a Galvanometer
If the dimensions and number of turns of the galvanometer and the value of H can be measured accurately the reduction factor can be calculated We shall suppose, however, that these data cannot be directly measured, and turn to another property of an electric current for a means of determining the reduction factor. Let i be a current which produces a deflexion φ in a galvanometer of which the reduction factor is k; then if it be used as a tangent instrument we have
and therefore,
If we can find by some other means the value of i, we can determine k by observing the deflexion φ which it produces. Now it has been found that when an electric current is allowed to pass through certain chemical compounds which are known as electrolytes, the passage of the current is accompanied by chemical decomposition. The process is called Electrolysis; the substance is resolved into two components called Ivns; these collect at the points at which the current enters and leaves the electrolytes respectively.
Moreover, it has been shown by Faraday ('Exp. Res.' ser. vii.) that the quantities of the ions deposited either at the kathode or the anode are proportional to the quantity of electricity which has passed. If this quantity be varied the quantity of the ions deposited varies in the same ratio. This is known as Faraday's law of electrolysis. We proceed to describe how to use this experimental result to determine the reduction factor of a galvanometer. Two copper plates are suspended in a beaker containing a solution of copper sulphate, by wires passing through a piece of dry wood or other insulating material which forms a covering to the beaker. The plates should be well cleaned before immersion by washing them with nitric acid, and then rinsing them with water, or by rubbing them with emery cloth, and then rinsing them with water. They must then be thoroughly dried. One of the plates must be carefully weighed to a milligramme. On being put into the solution this plate is connected to the negative pole - the zinc - of a constant battery, preferably a Daniell's cell, by means of copper wire; the other plate is connected with one electrode of the galvanometer. The positive pole of the battery is connected through a key with the other pole of the galvanometer, so that on making contact with the key the current flows from the copper of the battery round the galvanometer, through the electrolytic cell, depositing copper on the weighed plate, and finally passes to the zinc or negative pole of the battery. Since the galvanometer reading is most accurate when the deflexion is 45° (see p. 47), the battery should if possible be chosen so as to give about that deflexion. For this purpose a preliminary experiment may be necessary. It is also better if possible to attach the copper of the battery and the anode of the cell to two of the binding screws of a commutator, the other two being in connection with the galvanometer. By this means the current can easily be reversed in the galvanometer without altering the direction in which it flows in the cell, and thus readings of the deflexion on either side of the zero can be taken.
The connections are shown in fig. 58. B is the battery, the current leaves the voltameter V by the screw M, entering it at the binding screw N from the commutator C. This consists of four mercury cups, p, q, r, s, with two u-shaped pieces of copper as connectors. If p and s, q and r respectively be joined, the current circulates in one direction round the galvanometer; by joining p and q, r and s, the direction in the galvanometer is reversed. The cup r is connected with the positive pole of the battery B. Now make contact, and allow the current to flow through the circuit for fifteen minutes, observing the value of the deflexion at the end of each minute. If there be a commutator in the circuit as in the figure, adjust it so that the current flows in opposite directions during the two halves of the interval. Let φ be the mean of the deflexions observed. If the battery has been quite constant the deflexions observed will not have varied from minute to minute; in any case the deflexion must not have changed much during the interval. If any great variation shows itself, owing to changes in the battery or voltameter, the experiment must be commenced afresh. At the end of the fifteen minutes the weighed plate must be taken out of the solution, washed carefully, first under the tap, and then by pouring distilled water on it, and finally dried by being held in a current of hot dry air. It is then weighed carefully as before. It will be found to have increased in weight; let the increase be m grammes. Then the increase per second is m/(15 x 60), and since the electro-chemical equivalent of copper is 0.00328, the average value of the current in C.G.S. units (electro-magnetic measure) is
But if φ1, φ2 ..... φ15 be the readings of the deflexion, this average value of the current is also
And if φ1, φ2, &c., are not greatly different, this expression is very nearly equal to k tanφ, where φ is the average value of φ1, ..... φ15. We thus find
If the factor is so small that the copper deposited in fifteen minutes - m grammes - is too little to be determined accurately, the experiment must be continued in the same way for a longer period. It must be remembered that the mass m is to be expressed in grammes. Instead of using a glass beaker to hold the sulphate, it is sometimes convenient to make the containing vessel itself one of the electrodes. Thus a copper crucible may be used as cathode, like the platinum one in Poggendorff's voltameter; in this the sulphate is placed, and the anode may be a rod of copper which hangs down into it. This form is shown in the figure. We have already said that if the dimensions of the galvanometer coil, and the number of turns of the wire of which it is composed can be determined, the value of k can be calculated, provided that the value of H be known; or, on the other hand, H can be found from a knowledge of the dimensions, and of the value of k determined by experiment For if G be the galvanometer constant, r the mean radius, and n the number of turns, we have
Also
Whence
The current, which is determined by the observations given above, is measured in C.G.S. units. The value of k gives the current which deflects the needle 45°, measured also in the same units. To obtain the value in amperes we must multiply the result by 10, since the C.G.S. unit of current contains 10 amperes. Experiment. - Determine the reduction factor of the given galvanometer by electrolysis, comparing your result with that given by calculation. Enter the results thus -
|
||||||
Home  Electricity Electricity  Determination of the Reduction Factor of a Galvanometer Determination of the Reduction Factor of a Galvanometer |
||||||
Last Update: 2011-03-27