| Capacitors, Magnetic Circuits, and Transformers is a free introductory textbook on the physics of capacitors, coils, and transformers. See the editorial for more information.... |

|

Home  Energy Energy  The Law of Conservation of Energy The Law of Conservation of Energy |
||||||||






|
||||||||
The Law of Conservation of Energy
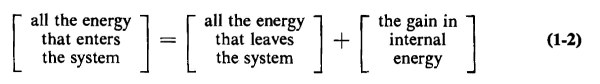
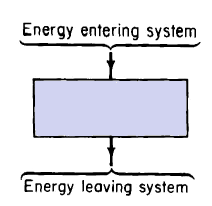
This law provides the basis for establishing relationships in all branches of engineering. It is also known as the First Law of Thermodynamics and postulates that energy can neither be created nor destroyed. Hence, for any system enclosed by boundaries as shown schematically in Fig. 1-2, the following relationship results from this law
In Eq. 1-2 the gain in the internal energy may be positive or negative; part or all of the internal energy may be reversible, or part or all of it may be irreversible. Reversible and irreversible processes
|
||||||||
Home  Energy Energy  The Law of Conservation of Energy The Law of Conservation of Energy |
||||||||
Last Update: 2011-02-16