| Basic Radio is a free introductory textbook on electronics based on tubes. See the editorial for more information.... |

|

Home  Electronic Devices Electronic Devices  Photoelectric Cells Photoelectric Cells  Introduction Introduction |
||||||
| See also: The Photo-Voltaic Effect | ||||||






|
||||||
|
Photoelectric CellsAuthor: J.B. Hoag
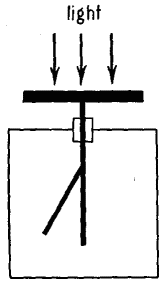
Light falls upon the horizontal zinc plate indicated by the heavy black bar near the top of the figure. Below this plate, in the box, is the gold leaf of an ordinary electroscope. When the light falls on the plate, and when the leaves of the electroscope are charged positively, there is no loss of electricity, but when the plate is negative, the leaves collapse. This shows that the photoelectrons are negatively charged. In other words, they are held by the attraction of a positive plate and are repelled from a negatively charged plate.
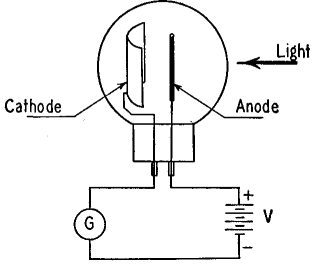
Figure 20 B shows a photoelectric tube in which the cathode is the surface upon which light is incident and from which electrons are emitted. The anode consists of a single straight wire and is charged with respect to the cathode by means of the battery V. The glass bulb surrounding these electrodes is evacuated, or at best contains only a small amount of gas. In the figure, G is a current-measuring instrument of a sensitive type known as a galvanometer. The deflection of the instrument is greater when more electrons leave the cathode, and vice versa.
|
||||||
Home  Electronic Devices Electronic Devices  Photoelectric Cells Photoelectric Cells  Introduction Introduction |
||||||
Last Update: 2010-11-21