| The ebook FEEE - Fundamentals of Electrical Engineering and Electronics is based on material originally written by T.R. Kuphaldt and various co-authors. For more information please read the copyright pages. |

|

Home  Experiments Experiments  DC Circuits DC Circuits  Potato battery Potato battery |
|






|
|
|
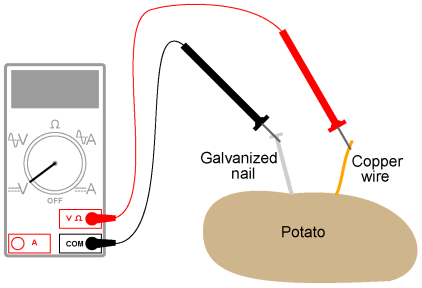
Potato batteryExperiment: Potato batteryPARTS AND MATERIALS
The basic experiment is based on the use of a potato, but many fruits and vegetables work as potential batteries! For the zinc electrode, a large galvanized nail works well. Nails with a thick, rough zinc texture are preferable to galvanized nails that are smooth.
CROSS-REFERENCES Lessons In Electric Circuits, Volume 1, chapter 11: "Batteries and Power Systems"
LEARNING OBJECTIVES
ILLUSTRATION
INSTRUCTIONS Push both the nail and the wire deep into the potato. Measure voltage output by the potato battery with a voltmeter. Now, wasn't that easy? Seriously, though, experiment with different metals, electrode depths, and electrode spacings to obtain the greatest voltage possible from the potato. Try other vegetables or fruits and compare voltage output with the same electrode metals. It can be difficult to power a load with a single "potato" battery, so don't expect to light up an incandescent lamp or power a hobby motor or do anything like that. Even if the voltage output is adequate, a potato battery has a fairly high internal resistance which causes its voltage to "sag" badly under even a light load. With multiple potato batteries connected in series, parallel, or series-parallel arrangement, though, it is possible to obtain enough voltage and current capacity to power a small load.
|
|
Home  Experiments Experiments  DC Circuits DC Circuits  Potato battery Potato battery |
|
Last Update: 2011-03-21