| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Thermometry and Expansion Thermometry and Expansion  Boiling Point of a Liquid Boiling Point of a Liquid |
||||||






|
||||||
Boiling Point of a Liquid
A liquid is usually said to boil at a temperature t when the pressure of its vapour at this temperature is equal to the external pressure p. But if the sides of the vessel be smooth and the liquid be quite free from dissolved air, or if it contain salts in solution, it will generally not boil till its temperature is higher than t.
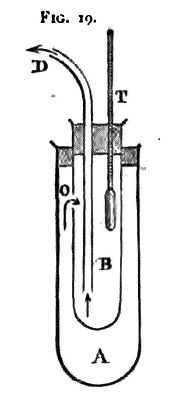
Hence the temperature of the vapour over a boiling liquid under a given pressure p, is a constant quantity under all circumstances, and is called the boiling point of the liquid under the pressure p. The hypsometer will serve to determine the boiling point of a liquid. In many cases, however, when the quantity of liquid obtainable is small, the apparatus described below is more convenient. The liquid is put into the outer glass tube (A). The inner tube (B), made of brass, is then restored to its place, as in fig. 17, and the whole placed on a sand bath and heated by a Bunsen burner.
When the liquid boils, the vapour will enter by the aperture o into the tube B, and will leave B by the glass tube D, which should be connected by a short piece of india-rubber tube with a condenser, to prevent the vapour entering the room. As the boiling continues, the thermometer will rise at first, but afterwards remain stationary. Enter this reading, and also the height of the barometer at the same time.
|
||||||
Home  Thermometry and Expansion Thermometry and Expansion  Boiling Point of a Liquid Boiling Point of a Liquid |
||||||
Last Update: 2011-03-27