| Basic Radio is a free introductory textbook on electronics based on tubes. See the editorial for more information.... |

|

Home  Electronic Devices Electronic Devices  Gas-Filled Tubes Gas-Filled Tubes  Ionizing Potentials Ionizing Potentials |
||||||






|
||||||
|
Ionization PotentialsAuthor: J.B. Hoag
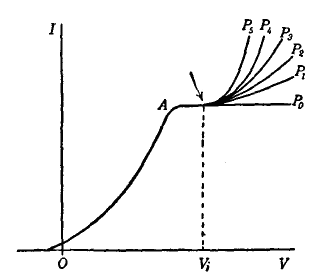
Ionizing potentials range between 3.88 and 24.5 volts. The following values are of interest in commercial tubes: mercury vapor, 10.39; argon, 15.7; nitrogen, 16.3; neon, 21.5; helium, 24.5. The number of atoms ionized per electron collision increases rapidly from a zero value, just below the ionizing potential, to a maximum value at 135 electron volts for mercury vapor, 140 for argon, 175 for nitrogen, 340 for neon, and 210 for helium. For electrons of still greater energy this ionization probability decreases slowly.
|
||||||
Home  Electronic Devices Electronic Devices  Gas-Filled Tubes Gas-Filled Tubes  Ionizing Potentials Ionizing Potentials |
||||||
Last Update: 2010-11-21