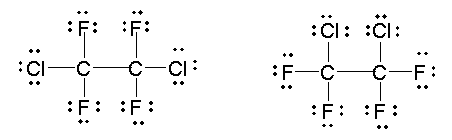| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Bonding Bonding  Covalent Bonding Covalent Bonding  Interpretation of Lewis Structures Interpretation of Lewis Structures |
|||||||






|
|||||||
Interpretation of Lewis StructuresAuthor: John Hutchinson
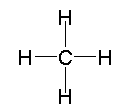
Before further developing our model of chemical bonding based on Lewis structures, we pause to consider the interpretation and importance of these structures. It is worth recalling that we have developed our model based on observations of the numbers of bonds formed by individual atoms and the number of valence electrons in each atom. In general, these structures are useful for predicting whether a molecule is expected to be stable under normal conditions. If we cannot draw a Lewis structure in which each carbon, oxygen, nitrogen, or halogen has an octet of valence electrons, then the corresponding molecule probably is not stable. Consideration of bond strengths and bond lengths enhances the model by revealing the presence of double and triple bonds in the Lewis structures of some molecules. At this point, however, we have observed no information regarding the geometries of molecules. For example, we have not considered the angles measured between bonds in molecules. Consequently, the Lewis structure model of chemical bonding does not at this level predict or interpret these bond angles (this will be considered here). Therefore, although the Lewis structure of methane is drawn as shown here.
This does not imply that methane is a flat molecule, or that the angles between CH bonds in methane is 90°. Rather, the structure simply reveals that the carbon atom has a complete octet of valence electrons in a methane molecule, that all bonds are single bonds, and that there are no non-bonding electrons. Similarly, one can write the Lewis structure for a water molecule in two apparently different ways, shown here.
However, it is very important to realize that these two structures are identical in the Lewis model, because both show that the oxygen atom has a complete octet of valence electrons, forms two single bonds with hydrogen atoms, and has two pairs of unshared electrons in its valence shell. In the same way, the two structures for Freon 114 shown here are also identical.
These two drawings do not represent different structures or arrangements of the atoms in the bonds. Finally, we must keep in mind that we have drawn Lewis structures strictly as a convenient tool for our understanding of chemical bonding and molecular stability. It is based on commonly observed trends in valence, bonding, and bond strengths. These structures must not be mistaken as observations themselves, however. As we encounter additional experimental observations, we must be prepared to adapt our Lewis structure model to fit these observations, but we must never adapt our observations to fit the Lewis model.
|
|||||||
Home  Bonding Bonding  Covalent Bonding Covalent Bonding  Interpretation of Lewis Structures Interpretation of Lewis Structures |
|||||||
Last Update: 2011-02-26