| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Inorganic Compounds Inorganic Compounds  Boron Boron  Borax Borax |
|||






|
|||
BoraxAuthor: Hans Lohninger
When heated to temperatures beyond 350°C borax decahydrate loses its crystal water and forms anhydrous borax. Molten borax (m.p. 743°C) forms a glass-like bead which can readily dissolve metal oxides, developing a characteristic color which can be used in analytical chemistry for the detection of certain metals ("borax bead"). Borax is easily converted to boric acid by reaction with hydrochloric acid:
Na2B4O7
Borax occurs naturally in evaporite deposits of seasonal lakes (California, Turkey, Chile, Tibet, Romania). The biggest borax producer is California. Most of the borax world production is used in the glass and ceramics industry (for ceramic glazes, optical glasses, and laboratory glassware).
|
|||
Home  Inorganic Compounds Inorganic Compounds  Boron Boron  Borax Borax |
|||
Last Update: 2011-03-16



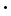 10H2O
10H2O
 4 H3BO3 + 2 NaCl + 5 H2O
4 H3BO3 + 2 NaCl + 5 H2O