| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Inorganic Compounds Inorganic Compounds  Silver Silver  Silver Chloride Silver Chloride |
|
| See also: Silver, Solubility Products, Silver Nitrate, Sodium Hydrogen Carbonate | |






|
|
Silver ChlorideAuthor: Hans Lohninger Silver chloride, AgCl, is a white crystalline solid which is well known for its low solubility in water. AgCl occurs naturally as the mineral chlorargyrite. Silver chloride converts to silver and chlorine, when subjected to sunlight or heating. AgCl adopts the fcc NaCl structure, in which Ag+ ions are surrounded by octahedrons of six chloride ligands. ChemistryAgCl dissolves in solutions containing chloride, cyanide, thiosulfate, or ammonium ions by forming complexes according to the following equations:
AgCl(s) + Cl-(aq) Due to the low solubility product of silver chloride, AgCl precipitates when a colorless silver nitrate solution ist mixed with a colorless sodium chloride solution:
Ag+(aq) + Cl-(aq)
The opaque white precipitate of AgCl quickly darkens on exposure to light. This reaction is a common test for the presence of chloride in a solution. The solubility product, Ksp, for AgCl is 1.8
UsageSilver chloride has a multitude of uses, some of them being listed below:
|
|
Home  Inorganic Compounds Inorganic Compounds  Silver Silver  Silver Chloride Silver Chloride |
|
Last Update: 2011-03-18


 AgCl2-(aq)
AgCl2-(aq)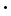 10-10; the solubility of AgCl in water is 1.9 mg/l at room temperature.
10-10; the solubility of AgCl in water is 1.9 mg/l at room temperature.