| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Molecules Molecules  The Kinetic Molecular Theory The Kinetic Molecular Theory  Boiling Points of Simple Hydrides Boiling Points of Simple Hydrides |
|||||||||||||||||||||||






|
|||||||||||||||||||||||
Boiling Points of Simple HydridesAuthor: John Hutchinson
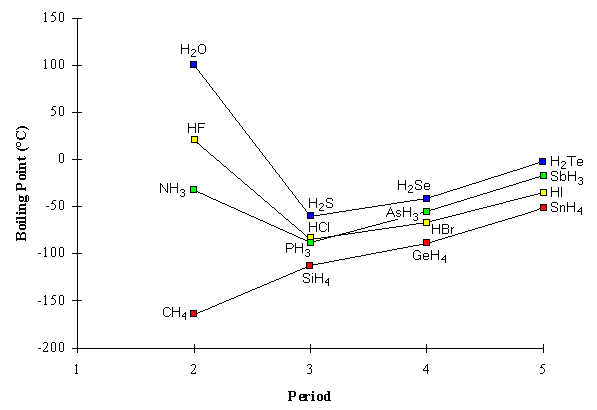
The postulates of the Kinetic Molecular Theory provide us a way to understand the relationship between molecular properties and the physical properties of bulk amounts of substance. As a distinct example of such an application, we now examine the boiling points of various compounds, focusing on hydrides of sixteen elements in the main group (Groups IV through VII). These are given here.
In tabular form, there are no obvious trends here, and therefore no obvious connection to the structure or bonding in the molecules. The data in the table are displayed in a suggestive form, however, in figure 4, the boiling point of each hydride is plotted according to which period (row) of the periodic table the main group element belongs. For example, the Period 2 hydrides (CH4, NH3, H2O, and HF) are grouped in a column to the left of the figure, followed by a column for the Period 3 hydrides (SiH4, PH3, H2S, HCl), etc. Now a few trends are more apparent. First, the lowest boiling points in each period are associated with the Group IV hydrides (CH4, SiH4, GeH4, SnH4), and the highest boiling points in each period belong to the Group VI hydrides (H2O, H2S, H2Se, H2Te). For this reason, the hydrides belonging to a single group have been connected in figure 4.
Second, we notice that, with the exceptions of NH3, H2O, and HF, the boiling points of the hydrides always increase in a single group as we go down the periodic table: for example, in Group IV, the boiling points increase in the order CH4<SiH4<GeH4<SnH4. Third, we can also say that the hydrides from Period 2 appear to have unusually high boiling points except for CH4, which as noted has the lowest boiling point of all. We begin our analysis of these trends by assuming that there is a relationship between the boiling points of these compounds and the structure and bonding in their molecules. Recalling our kinetic molecular model of gases and liquids, we recognize that a primary difference between these two phases is that the strength of the interaction between the molecules in the liquid is much greater than that in the gas, due to the proximity of the molecules in the liquid. In order for a molecule to leave the liquid phase and enter into the gas phase, it must possess sufficient energy to overcome the interactions it has with other molecules in the liquid. Also recalling the kinetic molecular description, we recognize that, on average, the energies of molecules increase with increasing temperature. We can conclude from these two statements that a high boiling point implies that significant energy is required to overcome intermolecular interactions. Conversely, a substance with a low boiling point must have weak intermolecular interactions, surmountable even at low temperature. In light of these conclusions, we can now look at figure 4 as directly (though qualitatively) revealing the comparative strengths of intermolecular interactions of the various hydrides. For example, we can conclude that, amongst the hydrides considered here, the intermolecular interactions are greatest between H2O molecules and weakest between CH4 molecules. We examine the three trends in this figure, described above, in light of the strength of intermolecular forces. First, the most dominant trend in the boiling points is that, within a single group, the boiling points of the hydrides increase as we move down the periodic table. This is true in all four groups in figure 4; the only exceptions to this trend are NH3, H2O, and HF. We can conclude that, with notable exceptions, intermolecular interactions increase with increasing atomic number of the central atom in the molecule. This is true whether the molecules of the group considered have dipole moments (as in Groups V, VI, and VII) or not (as in Group IV). We can infer that the large intermolecular attractions for molecules with large central atoms arises from the large number of charged particles in these molecules. This type of interaction arises from forces referred to as London forces or dispersion forces. These forces are believed to arise from the instantaneous interactions of the charged particles from one molecule with the charged particles in an adjacent molecule. Although these molecules may not be polar individually, the nuclei in one molecule may attract the electrons in a second molecule, thus inducing an instantaneous dipole in the second molecule. In turn, the second molecule induces a dipole in the first. Thus, two non-polar molecules can interact as if there were dipole-dipole attractions between them, with positive and negative charges interacting and attracting. The tendency of a molecule to have an induced dipole is called the polarizability of the molecule. The more charged particles there are in a molecule, the more polarizable a molecule is and the greater the attractions arising from dispersion forces will be. Second, we note that, without exception, the Group IV hydrides must have the weakest intermolecular interactions in each period. As noted above, these are the only hydrides that have no dipole moment. Consequently, in general, molecules without dipole moments have weaker interactions than molecules which are polar. We must qualify this carefully, however, by noting that the nonpolar SnH4 has a higher boiling point than the polar PH3 and HCl. We can conclude from these comparisons that the increased polarizability of molecules with heavier atoms can offset the lack of a molecular dipole.
Third, and most importantly, we note that the intermolecular attractions involving NH3, H2O, and HF must be uniquely and unexpectedly large, since their boiling points are markedly out of line with those of the rest of their groups. The common feature of these molecules is that they contain small atomic number atoms which are strongly electronegative, which have lone pairs, and which are bonded to hydrogen atoms. Molecules without these features do not have unexpectedly high boiling points. We can deduce from these observations that the hydrogen atoms in each molecule are unusually strongly attracted to the lone pair electrons on the strongly electronegative atoms with the same properties in other molecules. This intermolecular attraction of a hydrogen atom to an electronegative atom is referred to as hydrogen bonding. It is clear from our boiling point data that hydrogen bonding interactions are much stronger than either dispersion forces or dipole-dipole attractions.
|
|||||||||||||||||||||||
Home  Molecules Molecules  The Kinetic Molecular Theory The Kinetic Molecular Theory  Boiling Points of Simple Hydrides Boiling Points of Simple Hydrides |
|||||||||||||||||||||||
Last Update: 2011-04-07