| Problem 1 |
Using a styrofoam or rubber ball, prove to yourself that a tetrahedral arrangement provides the maximum separation of four points on the surface of the ball. Repeat this argument to find the expected arrangements for two, three, five, and six points on the surface of the ball. |
| Problem 2 |
Explain why arranging points on the surface of a sphere can be considered equivalent to arranging electron pairs about a central atom. |
| Problem 3 |
The valence shell electron pairs about the central atom in each of the molecules H2O, NH3, and CH4 are arranged approximately in a tetrahedron. However, only CH4 is considered a tetrahedral molecule. Explain why these statements are not inconsistent. |
| Problem 4 |
Explain how a comparison of the geometries of H2O and CH4 leads to a conclusion that lone pair electrons produce a greater repulsive effect than do bonded pairs of electrons. Give a physical reason why this might be expected. |
| Problem 5 |
Explain why the octet of electrons about each carbon atom in ethene, C2H4, are not arranged even approximately in a tetrahedron. |
| Problem 6 |
Assess the accuracy of the following reasoning and conclusions:
A trigonal bipyramid forms when there are five electron domains. If one ED is a lone pair, then the lone pair takes an equatorial position and the molecule has a seesaw geometry. If two EDs are lone pairs, we have to decide among the following options: both axial, both equatorial, or one axial and one equatorial. By placing both lone pairs in the axial positions, the lone pairs are as far apart as possible, so the trigonal planar structure is favored. |
| Problem 7 |
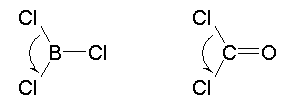 Assess the accuracy of the following reasoning and conclusions:
The Cl-X-Cl bond angles in the two molecules are identical, because the bond angle is determined by the repulsion of the two Cl atoms, which is identical in the two molecules.
Assess the accuracy of the following reasoning and conclusions:
The Cl-X-Cl bond angles in the two molecules are identical, because the bond angle is determined by the repulsion of the two Cl atoms, which is identical in the two molecules. |


 Bonding
Bonding  Molecular Geometry and Electron Domain Theory
Molecular Geometry and Electron Domain Theory  Problems
Problems





 Bonding
Bonding  Molecular Geometry and Electron Domain Theory
Molecular Geometry and Electron Domain Theory  Problems
Problems

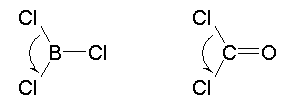 Assess the accuracy of the following reasoning and conclusions:
The Cl-X-Cl bond angles in the two molecules are identical, because the bond angle is determined by the repulsion of the two Cl atoms, which is identical in the two molecules.
Assess the accuracy of the following reasoning and conclusions:
The Cl-X-Cl bond angles in the two molecules are identical, because the bond angle is determined by the repulsion of the two Cl atoms, which is identical in the two molecules.