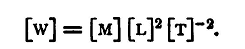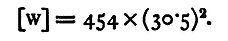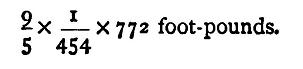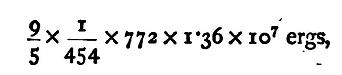| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Physical Measurements Physical Measurements  Conversion of Quantities Conversion of Quantities |
|||||






|
|||||
Conversion of Quantities
This method of converting from one system to another is only available when both systems are absolute and based on the same laws. If a quantity is expressed in arbitrary units, it must first be expressed in a unit belonging to some absolute system, and then the conversion factor can be calculated as above. For example: To express 15 Foot-pounds in Ergs. The foot-pound is not an absolute unit. We must first obtain the amount of work expressed in absolute units. Now, since g = 32.2 in British absolute units, 1 foot-pound = 32.2 foot-poundals (British absolute units).
We can now convert from foot-poundals to ergs. The dimensional equation is
Since
Substituting
we get
Hence
Sometimes neither of the units belongs strictly to an absolute system, although a change of the fundamental units alters the unit in question. For example: To find the Mechanical Equivalent of Heat in C.G.S. Centigrade Units, knowing that its Value for a Pound Fahrenheit Unit of Heat is 772 Foot-pounds. The mechanical equivalent of heat is the amount of work equivalent to one unit of heat. For the C.G.S. Centigrade unit of heat, it is, therefore,
This amount of heat is equivalent to
or the mechanical equivalent of heat in C.G.S. Centigrade units
If the agreement between scientific men as to the selection of fundamental units had been universal, a great deal of arithmetical calculation which is now necessary would have been avoided. There is some hope that in future one uniform system may be adopted, but even then it will be necessary for the student to be familiar with the methods of changing from one system to another in order to be able to avail himself of the results already published To form a -basis of calculation, tables showing the equivalents of the different fundamental units for the measurement of the same quantity are necessary. Want of space prevents our giving them here; we refer instead to Nos. 9-12 of the tables by Mr. S. Lupton, recently published. We take this opportunity of mentioning that we shall refer to the same work1 whenever we have occasion to notice the necessity for a table of constants for use in the experiments described.
|
|||||
Home  Physical Measurements Physical Measurements  Conversion of Quantities Conversion of Quantities |
|||||
Last Update: 2011-03-15