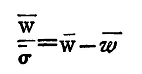| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Measurement of Mass and Specific Gravity Measurement of Mass and Specific Gravity  The Hydrostatic Balance The Hydrostatic Balance |
||||||||||||||||||||||||||






|
||||||||||||||||||||||||||
The Hydrostatic Balance
The specific gravity of a substance is determined by the hydrostatic balance by weighing the substance in air, and also in water. One scale pan is removed from the balance, and replaced by a pan suspended by shorter strings from the beam. This pan has a hook underneath, and from the hook the substance to be weighed is suspended by a piece of very fine wire. (1) To determine the Specific Gravity of a Solid heavier than Water. We must first make sure that the beam is horizontal when the balance is loaded only with the wire which is to carry the substance. Turn the key or lever gently to release the beam; the pointer will probably move sharply across the scale, showing that one pan is heavier than the other.
Do not counterpoise with weights which you may subsequently require in order to weigh the object. Hang the object whose specific gravity you require - a piece of copper suppose - by the fine wire from the hook above mentioned, and weigh it twice or three times by the method of oscillations. Let its weight be 11.378 grammes. Fill a vessel with distilled water, and bring it under the end of the beam so that the copper may dip completely into the water. Be careful that no air-bubbles adhere to the copper; if there be any, remove them by means of a small brush or feather, or a fibre of glass. It is well to use water that has been freed from dissolved air either by boiling or by means of an air-pump. Any very small bubbles not easily removable by mechanical means will then be dissolved by the water. Be careful also that the wire which supports the copper cuts the surface of the water only once; there is always a certain amount of sticking, due to surface tension between the wire and the surface of the water, and this is increased if a loose end of the wire be left which rises through the surface. To completely avoid the effect of surface tension the diameter of wire should not be greater than 0.004 inch. Weigh the copper in the water; it will probably be found that the pointer will not oscillate, but will come to rest almost immediately. Observe the resting-point, and by turning the key set the beam swinging again, and take another observation. Do this four times, and take the mean. Add some small weight, say goi gramme, to the weight, and observe another resting-point, and from these observations calculate, as in §12, the weight of the copper in water; it will be about 10.101 grammes. Observe at the same time the temperature of the water with a thermometer. Suppose it is 15°. Then it follows that the weight of the water displaced is 11.378-10.101 grammes, or 1.277 gramme. Now the specific gravity of a substance is equal to
In all cases, if we know the weight of a volume of water at t°, we can find its weight at 4°C., by dividing the weight at t° by the specific gravity of water at t°.
The specific gravity of water at t° may be taken from table (32). In this case, the weight of the equal volume of water at 15°C. is 1.277 gramme, and the specific gravity of water at 15° is 0.99917. The weight of the equal volume of water at 4°C.
Thus, the specific gravity of copper
It is well to pour the water into the beaker or vessel that is to hold it, before beginning the experiment, and leave it near the balance, so that it may acquire the temperature of the room. If greater accuracy be required, we must free the water used from air. This can be done by putting it under the receiver of an air-pump and exhausting, or by boiling the water for some time and then allowing it to cool. We have neglected the effect of the wire which is immersed in the water; we can, if we need, correct for this. We have also neglected the correction to the observed weight, which arises from the fact that the weights used displace some air, so that the observed weight in air is really the true weight minus the weight of air displaced. (2) To determine the Specific Gravity of a Solid lighter than Water. If we wish to find the specific gravity of a solid lighter than water, we must first weigh the light solid in air, then tie it on to a heavier solid, called a sinker, whose weight and specific gravity we know. The combination should be such that the whole will sink in water. Let w and σ be the weight in air, and the specific gravity of the light solid - a piece of wax, for instance - w', σ' corresponding quantities for the sinker, /w, /σ for the combination; w', /w the weights in water of the sinker and the combination respectively. Then, using C.G.S. units, w/σ represents the volume of the wax, w'/σ' that of the sinker, /w//σ that of the combination. Since the volume of the wax is equal to that of the combination minus that of the sinker, we get
But, with the proper temperature corrections,
and
or remembering that /w = w+w'
w, w', /w can each be observed, and thus the specific gravity of the wax determined. If it is convenient to tie the sinker so that it is immersed while the solid itself is out of the water, the following method is still simpler. Weigh the solid in air and let its weight be w. Attach the sinker below the solid, and weigh the combination with the former only immersed. Let the weight be W1. Raise the vessel containing the water so that the solid is immersed as well as the sinker, and let the weight be w2. Then, if the temperature of the water be t°, the specific gravity required
(3) To determine the Specific Gravity of a Liquid. Weigh a solid in air; let its weight be w. Weigh it in water; let the weight be w1. Weigh it in the liquid; let its weight be w2. The liquid must not act chemically on the solid, w - w1 is the weight of water displaced by the solid, and w - w2 is the weight of an equal volume of the liquid. Thus, the specific gravity of the liquid at 0°, if it expand by heat equally with water, and if the temperature of the two observations be the same, is the ratio of these weights. To find the specific gravity of the liquid at the temperature of the observation, τ° say, we must multiply this ratio by the specific gravity of water at the temperature at which the solid was weighed in water; let this be t°. Hence the specific gravity of the liquid at τ°
Experiments. (1) Determine the specific gravity of copper. (2) Determine the specific gravity of wax. Enter results as below, indicating how often each quantity has been observed. (1) Specific gravity of copper.
(2) Specific gravity of wax. Using the piece of copper (1) as sinker.
|
||||||||||||||||||||||||||
Home  Measurement of Mass and Specific Gravity Measurement of Mass and Specific Gravity  The Hydrostatic Balance The Hydrostatic Balance |
||||||||||||||||||||||||||
Last Update: 2011-03-27