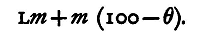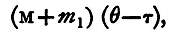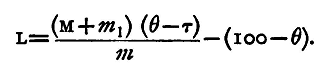| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Calorimetry Calorimetry  The Method of Mixture The Method of Mixture  Latent Heat of Steam Latent Heat of Steam |
||






|
||
Latent Heat of Steam
DEFINITION. - The heat required to convert a gramme of water at 100° C. into steam without altering its temperature is called the latent heat of steam at 100°C.
Let M be the mass of water in the calorimeter, m1 the water equivalent, τ the temperature initially, θ the common temperature after a mass m of steam has been passed in, L the latent heat of steam. The amount of heat given out by the steam in condensing to water, which is then cooled from 100° to 0°, is
The heat required to raise the calorimeter with the water from τ to θ is
and these two quantities of heat are equal. Hence
In practice various precautions are necessary. The steam coming directly from the boiler carries with it a large quantity of water, and moreover, in its passage through the various tubes some steam is condensed. Thus water would enter the calorimeter with the steam, and produce considerable error in the result This is avoided by surrounding all the tubes with jackets and drying the steam. To dry the steam a closed cylindrical vessel is employed, with two tubes entering it at the top and bottom, and a hole at the top, which can be closed by a cork carrying a thermometer. Inside this is a spiral of thin copper tubing; the spiral emerges at the top where a glass nozzle is attached by india-rubber tubing, and terminates at the bottom in a stop-cock. The continuation of the stop-cock and the tube at the top of the cylinder are attached by india-rubber tubing to the boiler; the tube at the bottom is connected with a condenser. Thus, on putting the top of the cylinder into connection with the boiler, a current of steam passes through the copper cylinder, raising it and the spiral inside to the temperature of 100°. If now we put the lower end of the spiral into communication with the boiler, the steam passes through the spiral, emerging through the nozzle. The spiral being kept hot at 100°, the steam inside it is freed from moisture and emerges from the nozzle in a dry state. The nozzle is connected with the spiral by means of a short piece of india-rubber tubing. This should be surrounded with cotton wool; the cylindrical heater is placed inside a wooden box, and surrounded with wool, or felt, or some other non-conducting substance. Sometimes it is more convenient to use the boiler itself to dry the steam; in this case the copper spiral is placed inside the boiler, from which one end emerges. The other end of the spiral inside the boiler is open above the level of the water. The steam, before emerging from the boiler, has to circulate through the spiral, and this dries it thoroughly. The calorimeter may conveniently take the form of a flask, or pear-shaped vessel, of thin copper, supported by silk threads inside another copper vessel. Its water equivalent must be determined in the same way as has been described in the section on specific heat (p. 216). In doing this, however, it must be remembered that the steam will probably raise the water to a temperature considerably higher than is the case in the determination of the specific heat of a metal. In like manner the temperature of the hot water used in finding the water equivalent should be considerably higher than that which was found most suitable in the previous experiments; it may with advantage be some 60° to 70°. Now water at this high temperature may cool considerably in being poured into the calorimeter, and care must be used to prevent loss of heat from this as far as possible. In allowing the steam to pass into the calorimeter the following method may be adopted: See that the steam passes freely from the nozzle, and note the temperature of the water in the calorimeter; pinch the india-rubber tube connecting the nozzle with the calorimeter for an instant, and immerse one end of the nozzle under the water, then allow the steam to flow until the temperature has risen about 20°. Raise the nozzle until its end is just above the level of the water in the calorimeter; again pinch the india-rubber tubing, stopping the flow of steam, and remove the calorimeter; note the highest point to which the temperature rises; this will be the value of θ, the common temperature. By pinching the tube as described above, the steam is prevented from blowing over the outer surface of the calorimeter. If, on the other hand, the tube be pinched and the flow stopped while the nozzle is under the water, the steam in the nozzle at the moment will be condensed, and the atmospheric pressure will drive some water up into the nozzle, and this will produce error. If the calorimeter is small there is some danger that the steam from the nozzle may flow directly on to the thermometer, and thus raise its temperature more than that of the surrounding water. This may be avoided by the use of a calorimeter of sufficient size. Another method of avoiding this error, and one which will lead to more accurate results, is the following, which has, however, the disadvantage of requiring more elaborate apparatus. The calorimeter contains a spiral tube of thin copper, ending in a closed vessel of the same material. This is completely surrounded by water, and the dry steam is passed through it instead of into the water. The water in the calorimeter is kept well stirred, and the heat given out by the steam in condensing is transmitted through the copper spiral and vessel to the water. The rise of temperature is noted as before, and when the temperature reaches its highest point, that is taken as the common temperature of the water, spiral, and calorimeter. The Heat absorbed by the spiral and vessel is determined with the water equivalent; the quantity of water in the spiral at the end gives the mass of steam condensed. (See Regnault's paper on the 'Latent Heat of Steam.' Memoires de l'Academie, T. xxi.) The calculation is proceeded with in the usual way. Experiment. - Determine the latent heat of steam.
|
||
Home  Calorimetry Calorimetry  The Method of Mixture The Method of Mixture  Latent Heat of Steam Latent Heat of Steam |
||
Last Update: 2011-03-27