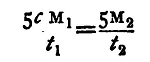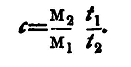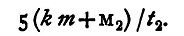| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Calorimetry Calorimetry  The Method of Cooling The Method of Cooling |
||||||






|
||||||
The Method of Cooling
A known weight of the liquid is put into a copper vessel with a thermometer. This is hung by means of silk threads, like the calorimeter, inside another copper vessel which is closed by a lid with a cork in it supporting the thermometer. The exterior vessel is kept in a large bath of water at a known temperature, the bath being kept well stirred. It is intended to be maintained at the temperature of the room throughout the experiment; the bath is simply to ensure this. A small stirrer should pass through the cork which holds the thermometer, to keep the liquid well stirred. The outer surface of the inner vessel and the inner surface of the outer should be coated with lampblack.
Allow the liquid to cool, and note the intervals taken by it to cool, through, say, each successive degree. If the rate of cooling is too rapid to allow this to be done, note the intervals for each 5°. or 10°, and calculate from these observations the mean rate of cooling for the range experimented on, say from 70° to 30°. Suppose we find that, on the average, it cools 3° in a minute. Then, if the liquid weigh 25 grammes and its specific heat be c, the quantity of heat which leaves it in one minute is 25 x 3 x c. Now empty the liquid out from the calorimeter and perform a similar experiment with water instead. The water should fill the calorimeter to the same level, and be raised to the same temperature as the liquid previously used. Let us now suppose that there are 32 grammes of water, and that the temperature of the water falls through 0.9 of a degree in one minute; thus the quantity of heat which escapes from the water per minute is 32 x 0.9 units. The quantity of heat radiated from one surface at a given temperature to another at a constant lower temperature depends solely on the nature and material of the surfaces and the temperature of the warmer.(1) In the two experiments described above, the surfaces are of the same nature; thus the rate at which heat escapes must be the same for the two experiments at the same temperatures,
We can get the result required from the observations more quickly thus: Observe the time it takes the temperature to fall, say, from 60° to 55° in the two cases; let it be t1 minutes and t2 minutes respectively. Then the fall of temperature per minute in the two cases respectively is 5/t1 and 5/t2. The amount of heat which is transferred in the first case
is 5cM1/t1, and in the second it is 5M2/t2, M1, M2 being the masses of the liquid and the water respectively. Thus
and
The effect of the vessel has hitherto been entirely neglected. Let k be its specific heat and m its mass, then in the first case the heat lost is
in the second it is
Thus
Instead of calculating the quantity km, we may find by experiment the water equivalent of the vessel and thermometer and use it instead of km. Experiment. - Determine the specific heat of the given liquid. Enter results thus: Weight of calorimeter: 15.13 g Weight of water: 10.94 g Weight of liquid 13.20 g Range of Time of cooling of Temperature Liquid Water Specific heat uncorrected 70-65 115 s 130 s 0.733 65-60 125 s 140 s 0.734 6o-55 150 s 170 s 0.733 55-50 107 s 190 s 0.736 Mean specific heat (uncorrected for calorimeter) = 0.734 Correction for calorimeter = -0.013 Specific heat of liquid = 0.721
|
||||||
Home  Calorimetry Calorimetry  The Method of Cooling The Method of Cooling |
||||||
Last Update: 2011-03-27