| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Physical Chemistry Physical Chemistry  Acid-Base Equilibrium Acid-Base Equilibrium  Strong Acids and Weak Acids Strong Acids and Weak Acids |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| See also: Dissociation Constant | |||||||||||||||||||||||||||||||||||||||||||||||||||||||






|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Strong and Weak AcidsAuthor: John Hutchinson
From the definition of an acid given in the Foundation, a typical acid can be written as HA, representing the hydrogen ion which will be donated and the rest of the molecule which will remain as a negative ion after the donation. The typical reaction of an acid in aqueous solution reacting with water can be written as
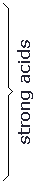
Equation 1 implies that a 0.1M solution of the acid HA in water should produce H3O+ ions in solution with a concentration of 0.1M. In fact, the concentration of H3O+ ions, [H3O+], can be measured by a variety of techniques. Chemists commonly use a measure of the H3O+ ion concentration called the pH, defined by: pH=-(log[H3O+]) We now observe the concentration [H3O+] produced by dissolving a variety of acids in solution at a concentration of 0.1M, and the results are tabulated in table 1.
Note that there are several acids listed for which [H3O+]=0.1M, and pH=1. This shows that, for these acids, the acid ionization is complete: essentially every acid molecule is ionized in the solution according to equation 1. However, there are other acids listed for which [H3O+] is considerably less than 0.1M and the pH is considerably greater than 1. For each of these acids, therefore, not all of the acid molecules ionize according to equation 1. In fact, it is clear in table 1 that in these acids the vast majority of the acid molecules do not ionize, and only a small percentage does ionize. From these observations, we distinguish two classes of acids: strong acids and weak acids. Strong acids are those for which nearly 100% of the acid molecules ionize, whereas weak acids are those for which only a small percentage of molecules ionize. There are seven strong acids listed in table 1. From many observations, it is possible to determine that these seven acids are the only commonly observed strong acids. The vast majority of all substances with acidic properties are weak acids. We seek to characterize weak acid ionization quantitatively and to determine what the differences in molecular properties are between strong acids and weak acids.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Home  Physical Chemistry Physical Chemistry  Acid-Base Equilibrium Acid-Base Equilibrium  Strong Acids and Weak Acids Strong Acids and Weak Acids |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Last Update: 2011-04-07


 H3O+(aq) + A-(aq)
H3O+(aq) + A-(aq)
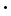 10-3
10-3