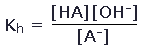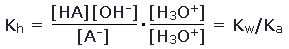| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Physical Chemistry Physical Chemistry  Acid-Base Equilibrium Acid-Base Equilibrium  Base Ionization, Neutralization and Hydrolysis of Salts Base Ionization, Neutralization and Hydrolysis of Salts |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||






|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Base Ionization, Neutralization and Hydrolysis of SaltsAuthor: John Hutchinson
We have not yet examined the behavior of base molecules in solution, nor have we compared the relative strengths of bases. We have defined a base molecule as one which accepts a positive hydrogen ion from another molecule. One of the most common examples is ammonia, NH3. When ammonia is dissolved in aqueous solution, the following reaction occurs:
Due to the lone pair of electrons on the highly electronegative N atom, NH3 molecules will readily attach a free hydrogen ion forming the ammonium ion NH4+. When we measure the concentration of OH- for various initial concentration of NH3 in water, we observe the results in table 6. We should anticipate that a base ionization equilibrium constant might exist comparable to the acid ionization equilibrium constant, and in table 6, we have also calculated the value of the function Kb defined as:
Given that we have dissolved a base in pure water, we might be surprised to discover the presence of positive hydrogen ions, H3O+, in solution, but a measurement of the pH for each of the solutions reveals small amounts. Base ionization is therefore quite analogous to acid ionization observed earlier. We now consider a comparison of the strength of an acid to the strength of a base. To do so, we consider a class of reactions called "neutralization reactions" which occur when we mix an acid solution with a base solution. Since the acid donates protons and the base accepts protons, we might expect, when mixing acid and base, to achieve a solution which is no longer acidic or basic. For example, if we mix together equal volumes of 0.1M HCl(aq) and 0.1M NaOH(aq), the following reaction occurs:
The resultant solution is simply a salt solution with NaCl dissolved in water. This solution has neither acidic nor basic properties, and the pH is 7; hence the acid and base have neutralized each other. In this case, we have mixed together a strong acid with a strong base. Since both are strong and since we mixed equal molar quantities of each, the neutralization reaction is essentially complete. We next consider mixing together a weak acid solution with a strong base solution, again with equal molar quantities of acid and base. As an example, we mix 100ml of 0.1M acetic acid (HA) solution with 100ml of 0.1M sodium hydroxide. In this discussion, we will abbreviate the acetic acid molecular formula CH3COOH as HA and the acetate ion CH3COO- as A-. The reaction of HA and NaOH is:
A-(aq) is the acetate ion in solution, formed when an acetic acid molecule donates the positive hydrogen ion. We have thus created a salt solution again, in this case of sodium acetate in water. Note that the volume of the combined solution is 200ml, so the concentration of sodium acetate (NaA) in solution is 0.050M. Unlike our previous NaCl salt solution, a measurement in this case reveals that the pH of the product salt solution is 9.4, so the solution is basic. Thus, mixing equal molar quantities of strong base with weak acid produces a basic solution. In essence, the weak acid does not fully neutralize the strong base. To understand this, we examine the behavior of sodium acetate in solution. Since the pH is greater than 7, then there is an excess of OH- ions in solution relative to pure water. These ions must have come from the reaction of sodium acetate with the water. Therefore, the negative acetate ions in solution must behave as a base, accepting positive hydrogen ions:
The reaction of an ion with water to form either an acid or a base solution is referred to as hydrolysis. From this example, the salt of a weak acid behaves as a base in water, resulting in a pH greater than 7.
To understand the extent to which the hydrolysis of the negative ion occurs, we need to know the equilibrium constant for this reaction. This turns out to be determined by the acid ionization constant for HA. To see this, we write the equilibrium constant for the hydrolysis of A- as
Multiplying numerator and denominator by [H3O+], we find that
Therefore, for the hydrolysis of acetate ions in solution, Kh = 5.8
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Home  Physical Chemistry Physical Chemistry  Acid-Base Equilibrium Acid-Base Equilibrium  Base Ionization, Neutralization and Hydrolysis of Salts Base Ionization, Neutralization and Hydrolysis of Salts |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Last Update: 2011-04-07


 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
 10-3
10-3