| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Bonding Bonding  Chemical Bonding and Molecular Energy Levels Chemical Bonding and Molecular Energy Levels  Ionization Energies of Diatomic Molecules Ionization Energies of Diatomic Molecules |
|||||||||
| See also: Bonding and Non-Bonding in Diatomic Molecules, Diatomic Molecules | |||||||||






|
|||||||||
Ionization Energies of Diatomic MoleculeAuthor: Hans Lohninger
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy. This is the energy required to remove an electron, in this case, from a molecule: H2 (g) --> H2+(g) + e-(g) The measured ionization energy of H2 is 1488 kJ/mol. This number is primarily important in comparison to the ionization energy of a hydrogen atom, which is 1312 kJ/mol. Therefore, it requires more energy to remove an electron from the hydrogen molecule than from the hydrogen atom, so we can conclude that the electron has a lower energy in the molecule. If we attempt to pull the atoms apart, we must raise the energy of the electron. Hence, energy is required to break the bond, so the molecule is bound. We conclude that a bond is formed when the energy of the electrons in the molecule is lower than the energy of the electrons in the separated atoms. This conclusion seems consistent with our previous view of shared electrons in bonding molecular orbitals. As a second example, we consider the nitrogen molecule, N2. We find that the ionization energy of molecular nitrogen is 1503 kJ/mol, and that of atomic nitrogen is 1402 kJ/mol. Once again, we conclude that the energy of the electrons in molecular nitrogen is lower than that of the electrons in the separated atoms, so the molecule is bound. As a third example, we consider fluorine, F2. In this case, we find that the ionization energy of molecular fluorine is 1515 kJ/mol, which is smaller than the ionization energy of a fluorine atom, 1681 kJ/mol. This seems inconsistent with the bonding orbital concept we have developed above, which states that the electrons in the bond have a lower energy than in the separated atoms. If the electron being ionized has a higher energy in F2 than in F, why is F2 a stable molecule? Apparently, we need a more complete description of the molecular orbital concept of chemical bonding. To proceed further, we compare bond energies in several molecules. Recall that the bond energy (or bond strength) is the energy required to separate the bonded atoms. We observe that the bond energy of N2 is 956 kJ/mol. This is very much larger than the bond energy of H2, 458 kJ/mol, and of F2, which is 160 kJ/mol. We can account for the unusually strong bond in nitrogen using both our valence shell electron pair sharing model and our electron orbital descriptions. A nitrogen atom has three unpaired electrons in its valence shell, because the three 2p electrons distribute themselves over the three 2p orbitals, each oriented along a different axis. Each of these unpaired electrons is available for sharing with a second nitrogen atom. The result, from valence shell electron pair sharing concepts, is that three pairs of electrons are shared between two nitrogen atoms, and we call the bond in N2 a “triple bond.” It is somewhat intuitive that the triple bond in N2 should be much stronger than the single bond in H2 or in F2. Now consider the molecular orbital description of bonding in N2. Each of the three 2p atomic orbitals in each nitrogen atom must overlap to form a bonding molecular orbital, if we are to accommodate three electron pairs. Each 2p orbital is oriented along a single axis. One 2p orbital from each atom is oriented in the direction of the other atom, that is, along the bond axis. When these two atomic orbitals overlap, they form a molecular orbital which has the symmetry of a cylinder and which is therefore a σ orbital. Of course, they also form a σ*orbital. The two electrons are then paired in the bonding orbital.
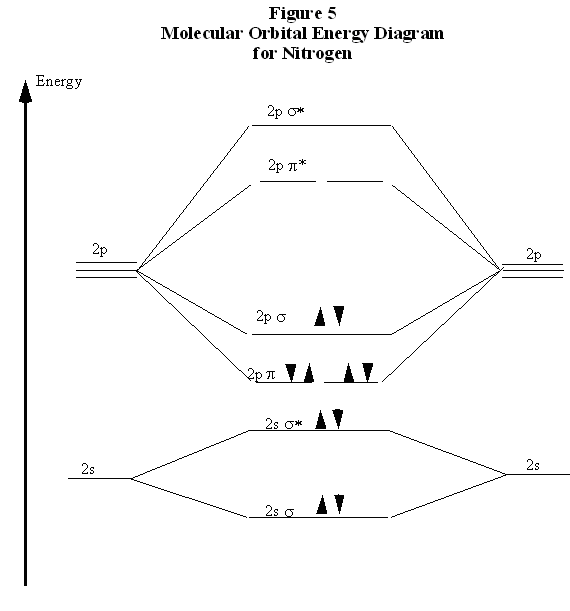
The other two 2p orbitals on each nitrogen atom are perpendicular to the bond axis. The constructive overlap between these orbitals from different atoms must therefore result in a molecular orbital somewhat different that what we have discussed before. As shown in Fig. 4, the molecular orbital which results now does not have the symmetry of a cylinder, and in fact, looks something more like a cylinder cut into two pieces. This we call a π orbital. There are two such π orbitals since there are two sets of p orbitals perpendicular to the bond axis. Figure 4 also shows that an anti-bonding orbital is formed from the destructive overlap of 2p orbitals, and this is called a π* orbital. There are also two π* orbitals formed from destructive overlap of 2p orbitals. In N2, the three shared electron pairs are thus in a single σ orbital and in two π orbitals. Each of these orbitals is a bonding orbital, therefore all six electrons have their energy lowered in comparison to the separated atoms. This is depicted in Fig. 5 in what is called a “molecular orbital energy diagram.” Each pair of atomic orbitals, one from each atom, is overlapped to form a bonding and an anti-bonding orbital. The three 2p orbitals from each atom form one σ and σ* pair and two π and π* pairs. The lowering of the energies of the electrons in the σand π orbitals is apparent. The ten n=2 electrons from the nitrogen atoms are then placed pairwise, in order of increasing energy, into these molecular orbitals. Note that, in agreement with the Pauli Exclusion Principle, each pair in a single orbital consists of one spin up and one spin down electron.
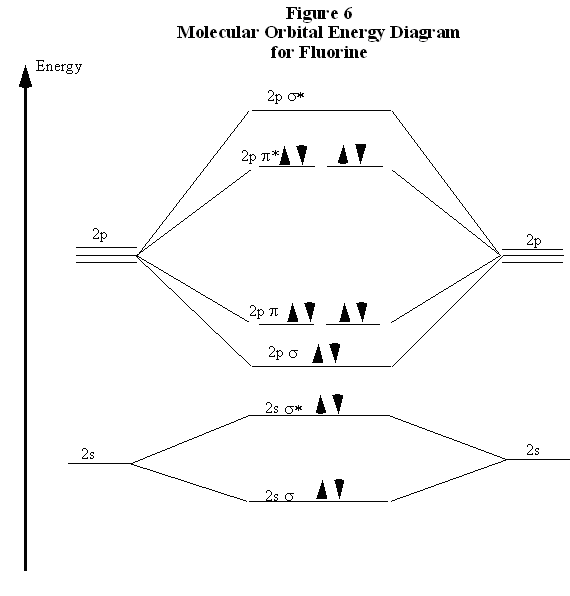
Recall now that we began the discussion of bonding in N2 because of the curious result that the ionization energy of an electron in F2 is less than that of an electron in an F atom. By comparing the molecular orbital energy level diagrams for N2 and F2 we are now prepared to answer this puzzle. There are five p electrons in each fluorine atom. These ten electrons must be distributed over the molecular orbitals whose energies are shown in Fig. 6. (Note that the ordering of the bonding 2p orbitals differ between N2 and F2.) We place two electrons in the σ orbital, four more in the two π orbitals, and four more in the two π* orbitals. Overall, there are six electrons in bonding orbitals and four in anti-bonding orbitals. Since F2 is a stable molecule, we must conclude that the lowering of energy for the electrons in the bonding orbitals is greater than the raising of energy for the electrons in the antibonding orbitals. Overall, this distribution of electrons is, net, equivalent to having two electrons paired in a single bonding orbital.
This also explains why the ionization energy of F2 is less than that of an F atom. The electron with the highest energy requires the least energy to remove from the molecule or atom. The molecular orbital energy diagram in Fig. 6 clearly shows that the highest energy electrons in F2 are in anti-bonding orbitals. Therefore, one of these electrons is easier to remove than an electron in an atomic 2p orbital, because the energy of an anti-bonding orbital is higher than that of the atomic orbitals. (Recall that this is why an anti-bonding orbital is, indeed, anti-bonding.) Therefore, the ionization energy of molecular fluorine is less than that of atomic fluorine. This clearly demonstrates the physical reality and importance of the anti-bonding orbitals. A particularly interesting case is the oxygen molecule, O2. In completing the molecular orbital energy level diagram for oxygen, we discover that we must decide whether to pair the last two electrons in the same 2pπ* orbital, or whether they should be separated into different 2pπ* orbitals. To determine which, we note that oxygen molecules are paramagnetic, meaning that they are strongly attracted to a magnetic field. To account for this paramagnetism, we recall that electron spin is a magnetic property. In most molecules, all electrons are paired, so for each “spin up” electron there is a “spin down” electron and their magnetic fields cancel out. When all electrons are paired, the molecule is diamagnetic meaning that it responds only weakly to a magnetic field. If the electrons are not paired, they can adopt the same spin in the presence of a magnetic field. This accounts for the attraction of the paramagnetic molecule to the magnetic field. Therefore, for a molecule to be paramagnetic, it must have unpaired electrons. The correct molecular orbital energy level diagram for an O2 molecule is shown in Fig. 7.
In comparing these three diatomic molecules, we recall that N2 has the strongest bond, followed by O2 and F2. We have previously accounted for this comparison with Lewis structures, showing that N2 is a triple bond, O2 is a double bond, and F2 is a single bond. The molecular orbital energy level diagrams in Figs. 5 to 7 cast a new light on this analysis. Note that, in each case, the number of bonding electrons in these molecules is eight. The difference in bonding is entirely due to the number of antibonding electrons: 2 for N2, 4 for O2, and six for F2. Thus, the strength of a bond must be related to the relative numbers of bonding and antibonding electrons in the molecule. Therefore, we now define the bond order as BondOrder = 1/2*(# bonding electrons - # antibonding electrons) Note that, defined this way, the bond order for N2 is 3, for O2 is 2, and for F2 is 1, which agrees with our conclusions from Lewis structures. We conclude that we can predict the relative strengths of bonds by comparing bond orders.
|
|||||||||
Home  Bonding Bonding  Chemical Bonding and Molecular Energy Levels Chemical Bonding and Molecular Energy Levels  Ionization Energies of Diatomic Molecules Ionization Energies of Diatomic Molecules |
|||||||||
Last Update: 2011-05-26