| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Bonding Bonding  Chemical Bonding and Molecular Energy Levels Chemical Bonding and Molecular Energy Levels  Bonding and Non-Bonding in Diatomic Molecules Bonding and Non-Bonding in Diatomic Molecules |
|||
| See also: Ionization Energies of Diatomic Molecule, Diatomic Molecules | |||






|
|||
Bonding and Non-Bonding in Diatomic MoleculesAuthor: Hans Lohninger
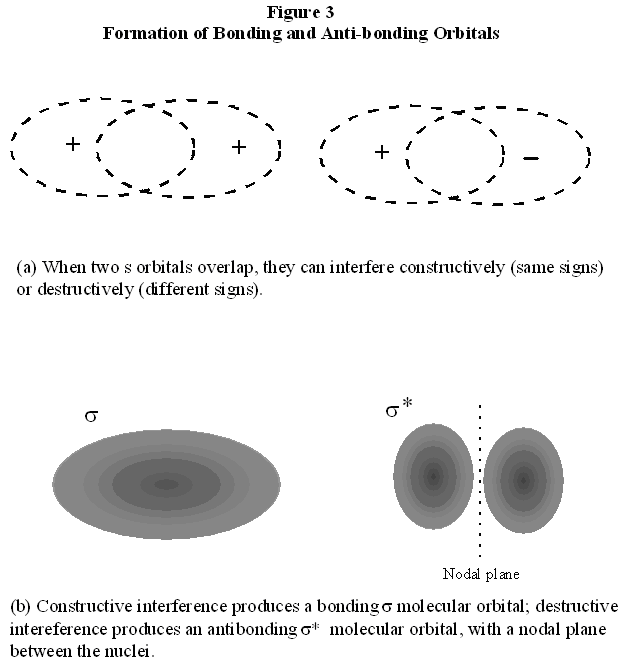
We now consider molecules with more than one electron. These are illustrated most easily by diatomic molecules (molecules with only two atoms) formed by like atoms, beginning with the hydrogen molecule, H2. The most direct experimental observation of a chemical bond is the amount of energy required to break it. This is called the bond energy, or somewhat less precisely, the bond strength. Experimentally, it is observed that the bond energy of the hydrogen molecule H2 is 458 kJ/mol. By contrast, the bond energy of the H2+ molecular ion is 269 kJ/mol. Therefore, the bond in H2 is stronger than the bond in H2+. Thus, the pair of shared electrons in H2 generates a stronger attractive force than does the single electron in H2+. Before deducing an explanation of this in terms of electron orbitals, we first recall the valence shell electron pair description of the bonding in H2. Each hydrogen atom has a single electron. By sharing these two electrons, each hydrogen atom can fill its valence shell, attaining the electron configuration of helium. How does this translate into the electron orbital picture of electron sharing that we have just described for the H2+ molecular ion? There are two ways to deduce the answer to this question, and, since they are both useful, we will work through them both. The first way is to imagine that we form an H2 molecule by starting with an H2+ molecular ion and adding an electron to it. As a simple approximation, we might imagine that the first electron’s probability distribution (its orbital) is not affected by the addition of the second electron. The second electron must have a probability distribution describing its location in the molecule as well. We recall that, in atoms, it is possible to put two electrons into a single electron orbital, provided that the two electrons have opposite values of the spin quantum number, ms. Therefore, we expect this to be true for molecules as well, and we place the added second electron in H2 into the same σ orbital as the first. This results in two electrons in the region between the two nuclei, thus adding to the force of attraction of the two nuclei into the bond. This explains our observation that the bond energy of H2 is almost (although not quite) twice the bond energy of H2+. The second way to understand the electron orbital picture of H2 is to imagine that we form the molecule by starting with two separated hydrogen atoms. Each of these atoms has a single electron in a 1s orbital. As the two atoms approach one another, each electron orbital is polarized in the direction of the other atom. Once the atoms are close enough together, these two orbitals become superimposed. Now we must recall that these orbitals describe the wave-like motion of the electron, so that, when these two wave functions overlap, they must interfere, either constructively or destructively. In Fig. 3, we see the consequences of constructive and destructive interference. We can deduce that, in H2 the electron orbitals from the atoms must constructively interfere, because that would increase the electron probability in the region between the nuclei, resulting in bonding as before. Therefore, the σ molecular orbital describing the two electrons in H2 can be understood as resulting from the constructive overlap of two atomic 1s electron orbitals. We now add to our observations of diatomic molecules by noting that, of the diatomic molecules formed from like atoms of the first ten elements, H2, Li2, B2, C2, N2, O2, and F2 are stable molecules with chemical bonds, whereas He2, Be2, and Ne2 are not bound. In examining the electron configurations of the atoms of these elements, we discover a correspondence with which diatomic molecules are bound and which ones are not. H, Li, B, N, and F all have odd numbers of electrons, so that at least one electron in each atom is unpaired. By contrast, He, Be, and Ne all have even numbers of electrons, none of which are unpaired. The other atoms, C and O both have an even number of electrons. However, as deduced in our understanding of the electron configurations in atoms, electrons will, when possible, distribute themselves into different orbitals of the same energy so as to reduce the effect of their mutual repulsion. Thus, in C and O, there are three 2p orbitals into which 2 and 4 electrons are placed, respectively. Therefore, in both atoms, there are two unpaired electrons. We conclude that bonds will form between atoms if and only if there are unpaired electrons in these atoms. In H2, the unpaired electrons from the separated atoms become paired in a molecular orbital formed from the overlap of the 1s atomic electron orbitals. In the case of a hydrogen atom, then, there are of course no paired electrons in the atom to worry about. In all other atoms, there certainly are paired electrons, regardless of whether there are or are not unpaired electrons. For example, in a lithium atom, there are two paired electrons in a 1s orbital and an unpaired electron in the 2s orbital. To form Li2, the unpaired electron from each atom can be placed into a molecular orbital formed from the overlap of the 2s atomic electron orbitals. However, what becomes of the two electrons paired in the 1s orbital in a Li atom during the bonding of Li2? To answer this question, we examine He2, in which each atom begins with only the two 1s electrons. As we bring the two He atoms together from a large distance, these 1s orbitals should become polarized, as in the hydrogen atom. When the polarized 1s orbitals overlap, constructive interference will again result in a σ molecular orbital, just as in H2. Yet, we observe that He2 is not a stable bound molecule. The problem which prevents bonding for He2 arises from the Pauli Exclusion Principle: only two of the four electrons in He2 can be placed into this σ bonding molecular orbital. The other two must go into a different orbital with a different probability distribution. To deduce the form of this new orbital, we recall that the bonding orbital discussed so far arises from the constructive interference of the atomic orbitals, as shown in Fig. 3. We could, instead, have assumed destructive interference of these orbitals. Destructive interference of two waves eliminates amplitude in the region of overlap of the waves, also shown in Fig. 3. In the case of the atomic orbitals, this means that the molecular orbital formed from destructive interference decreases probability for the electron to be between in the nuclei. Therefore, it increases probability for the electron to be outside the nuclei, as in Fig. 1a. As discussed there, this arrangement for the electron does not result in bonding; instead, the nuclei repel each other and the atoms are forced apart. This orbital is thus called an anti-bonding orbital. This orbital also has the symmetry of a cylinder along the bond axis, so it is also a σorbital; to indicate that it is an anti-bonding orbital, we designate it with an asterisk, σ*.
In He2, both the bonding and the anti-bonding orbitals must be used in order to accommodate four electrons. The two electrons in the bonding orbital lower the energy of the molecule, but the two electrons in the anti-bonding orbital raise it. Since two He atoms will not bind together, then the net effect must be that the anti-bonding orbital more than offsets the bonding orbital. We have now deduced an explanation for why the paired electrons in an atom do not contribute to bonding. Both bonding and anti-bonding orbitals are always formed when two atomic orbitals overlap. When the electrons are already paired in the atomic orbitals, then there are too many electrons for the bonding molecular orbital. The extra electrons must go into the anti-bonding orbital, which raises the energy of the molecule, preventing the bond from forming. Returning to the Li2 example discussed above, we can develop a simple picture of the bonding. The two 1s electrons from each atom do not participate in the bonding, since the anti-bonding more than offsets the bonding. Thus, the paired “core” electrons remain in their atomic orbitals, unshared, and we can ignore them in describing the bond. The bond is formed due to overlap of the 2s orbitals and sharing of these electrons only. This is also consistent with our earlier view that the core electrons are closer to the nucleus, and thus unlikely to be shared by two atoms. The model we have constructed seems to describe fairly well the bonding in the bound diatomic molecules listed above. For example, in a fluorine atom, the only unpaired electron is in a 2p orbital. Recall that a 2p orbital has two lobes, directed along one axis. If these lobes are assumed to lie along the axis between the two nuclei in F2, then we can overlap them to form a bonding orbital. Placing the two unpaired electrons into this orbital then results in a single shared pair of electrons and a stable molecular bond.
|
|||
Home  Bonding Bonding  Chemical Bonding and Molecular Energy Levels Chemical Bonding and Molecular Energy Levels  Bonding and Non-Bonding in Diatomic Molecules Bonding and Non-Bonding in Diatomic Molecules |
|||
Last Update: 2011-05-26