| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Bonding Bonding  Molecular Structure and Physical Properties Molecular Structure and Physical Properties  Compounds of Groups I and II Compounds of Groups I and II |
||||||||||||||||||||||||||||||||||||||||||||||






|
||||||||||||||||||||||||||||||||||||||||||||||
Compounds of Groups I and IIAuthor: John Hutchinson
We begin by analyzing compounds formed from elements from Groups I and II (e.g. sodium and magnesium). These compounds are not currently part of our Lewis structure model. For example, Sodium, with a single valence electron, is unlikely to gain seven additional electrons to complete an octet. Indeed, the common valence of the alkali metals in Group I is 1, not 7, and the common valence of the alkaline earth metals is 2, not 6. Thus, our current model of bonding does not apply to elements in these groups. To develop an understanding of bonding in these compounds, we focus on the halides of these elements. In table 1, we compare physical properties of the chlorides of elements in Groups I and II to the chlorides of the elements of Groups IV, V, and VI, and we see enormous differences. All of the alkali halides and alkaline earth halides are solids at room temperature and have melting points in the hundreds of degrees centigrade. The melting point of NaCl is 808°C, for example. By contrast, the melting points of the non-metal halides from Periods 2 and 3, such as CCl4, PCl3, and SCl2, are below 0°C, so that these materials are liquids at room temperature. Furthermore, all of these compounds have low boiling points, typically in the range of 50°C to 80°C.
Second, the non-metal halide liquids are electrical insulators, that is, they do not conduct an electrical current. By contrast, when we melt an alkali halide or alkaline earth halide, the resulting liquid is an excellent electrical conductor. This indicates that these molten compounds consist of ions, whereas the non-metal halides do not. We must conclude that the bonding of atoms in alkali halides and alkaline earth halides differs significantly from bonding in non-metal halides. We need to extend our valence shell electron model to account for this bonding, and in particular, we must account for the presence of ions in the molten metal halides. Consider the prototypical example of NaCl. We have already deduced that Cl atoms react so as to form a complete octet of valence shell electrons. Such an octet could be achieved by covalently sharing the single valence shell electron from a sodium atom. However, such a covalent sharing is clearly inconsistent with the presence of ions in molten sodium chloride. Furthermore, this type of bond would predict that NaCl should have similar properties to other covalent chloride compounds, most of which are liquids at room temperature. By contrast, we might imagine that the chlorine atom completes its octet by taking the valence shell electron from a sodium atom, without covalent sharing. This would account for the presence of Na+ and Cl- ions in molten sodium chloride. In the absence of a covalent sharing of an electron pair, though, what accounts for the stability of sodium chloride as a compound? It is relatively obvious that a negatively charged chloride ion will be attracted electrostatically to a positively charged sodium ion. We must also add to this model, however, the fact that individual molecules of NaCl are not generally observed at temperatures less than 1465°C, the boiling point of sodium chloride. Note that, if solid sodium chloride consists of individual sodium ions in proximity to individual chloride ions, then each positive ion is not simply attracted to a single specific negative ion but rather to all of the negative ions in its near vicinity. Hence, solid sodium chloride cannot be viewed as individual NaCl molecules, but must be viewed rather as a lattice of positive sodium ions interacting with negative chloride ions. This type of “ionic” bonding, which derives from the electrostatic attraction of interlocking lattices of positive and negative ions, accounts for the very high melting and boiling points of the alkali halides.
This indicates explicitly that the bonding is due to positive-negative ion attraction, and not due to sharing of an electron pair. The only sense in which the Na+ ion has obeyed an octet rule is perhaps that, in having emptied its valence shell of electrons, the remaining outer shell of electrons in the ion has the same octet as does a neon atom. We must keep in mind, however, that the positive sodium ion is attracted to many negative chloride ions, and not just the single chloride ion depicted in the Lewis structure.
|
||||||||||||||||||||||||||||||||||||||||||||||
Home  Bonding Bonding  Molecular Structure and Physical Properties Molecular Structure and Physical Properties  Compounds of Groups I and II Compounds of Groups I and II |
||||||||||||||||||||||||||||||||||||||||||||||
Last Update: 2011-02-20


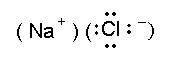 We can now draw modified Lewis structures to account for ionic bonding, but these are very different from our previous drawings. Sodium chloride can be represented as shown at the right.
We can now draw modified Lewis structures to account for ionic bonding, but these are very different from our previous drawings. Sodium chloride can be represented as shown at the right.