| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Physical Chemistry Physical Chemistry  Reaction Equilibrium in the Gas Phase Reaction Equilibrium in the Gas Phase  Introduction Introduction |
|||






|
|||
IntroductionAuthor: John Hutchinson
In beginning our study of the reactions of gases, we will assume a knowledge of the physical properties of gases as described by the Ideal Gas Law and an understanding of these properties as given by the postulates and conclusions of the Kinetic Molecular Theory. We assume that we have developed a dynamic model of phase equilibrium in terms of competing rates. We will also assume an understanding of the bonding, structure, and properties of individual molecules. In performing stoichiometric calculations, we assume that we can calculate the amount of product of a reaction from the amount of the reactants we start with. For example, if we burn methane gas, CH4(g), in excess oxygen, the reaction
occurs, and the number of moles of CO2(g) produced is assumed to equal the number of moles of CH4 (g) we start with. From our study of phase transitions we have learned the concept of equilibrium. We observed that, in the transition from one phase to another for a substance, under certain conditions both phases are found to coexist, and we refer to this as phase equilibrium. It should not surprise us that these same concepts of equilibrium apply to chemical reactions as well. In the reaction [1], therefore, we should examine whether the reaction actually produces exactly one mole of CO2 for every mole of CH4 we start with or whether we wind up with an equilibrium mixture containing both CO2 and CH4. We will find that different reactions provide us with varying answers. In many cases, virtually all reactants are consumed, producing the stoichiometric amount of product. However, in many other cases, substantial amounts of reactant are still present when the reaction achieves equilibrium, and in other cases, almost no product is produced at equilibrium. Our goal will be to understand, describe and predict the reaction equilibrium. An important corollary to this goal is to attempt to control the equilibrium. We will find that varying the conditions under which the reaction occurs can vary the amounts of reactants and products present at equilibrium. We will develop a general principle for predicting how the reaction conditions affect the amount of product produced at equilibrium.
|
|||
Home  Physical Chemistry Physical Chemistry  Reaction Equilibrium in the Gas Phase Reaction Equilibrium in the Gas Phase  Introduction Introduction |
|||
Last Update: 2011-03-19


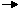 CO2(g) + 2H2O(g)
CO2(g) + 2H2O(g)