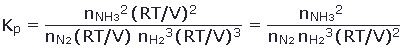| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Physical Chemistry Physical Chemistry  Reaction Equilibrium in the Gas Phase Reaction Equilibrium in the Gas Phase  Changes in Equilibrium and Le Châtelier's Principle Changes in Equilibrium and Le Châtelier's Principle |
|||||||






|
|||||||
Changes in Equilibrium and Le Châtelier's PrincipleAuthor: John Hutchinson
One of our goals at the outset was to determine whether it is possible to control the equilibrium which occurs during a gas reaction. We might want to force a reaction to produce as much of the products as possible. In the alternative, if there are unwanted by-products of a reaction, we might want conditions which minimize the product. We have observed that the amount of product varies with the quantities of initial materials and with changes in the temperature. Our goal is a systematic understanding of these variations. A look back at table 1 and table 2 shows that the equilibrium pressure of the product of the reaction increases with increasing the initial quantity of reaction. This seems quite intuitive. Less intuitive is the variation of the equilibrium pressure of the product of this reaction with variation in the volume of the container, as shown in table 3. Note that the pressure of NH3 decreases by more than a factor of ten when the volume is increased by a factor of ten. This means that, at equilibrium, there are fewer moles of NH3 produced when the reaction occurs in a larger volume. To understand this effect, we rewrite the equilibrium constant in equation 8 to explicit show the volume of the container. This is done by applying Dalton's Law of Partial Pressures, so that each partial pressure is given by the Ideal Gas Law:
Therefore,
This form of the equation makes it clear that, when the volume increases, the left side of the equation decreases. This means that the right side of the equation must decrease also, and in turn, nNH3 must decrease while nN2 and nH2 must increase. The equilibrium is thus shifted from products to reactants when the volume increases for this reaction. The effect of changing the volume must be considered for each specific reaction, because the effect depends on the stoichiometry of the reaction. One way to determine the consequence of a change in volume is to rewrite the equilibrium constant as we have done in equation 11. Finally, we consider changes in temperature. We note that Kp increases with T for endothermic reactions and decreases with T for exothermic reactions. As such, the products are increasingly favored with increasing temperature when the reaction is endothermic, and the reactants are increasingly favored with increasing temperature when the reaction is exothermic. On reflection, we note that when the reaction is exothermic, the reverse reaction is endothermic. Putting these statements together, we can say that the reaction equilibrium always shifts in the direction of the endothermic reaction when the temperature is increased. All of these observations can be collected into a single unifying concept known as Le Châtelier's Principle.
This statement is best understood by reflection on the types of "stresses" we have considered in this section. When a reactant is added to a system at equilibrium, the reaction responds by consuming some of that added reactant as it establishes a new equilibrium. This offsets some of the stress of the increase in reactant. When the temperature is raised for a reaction at equilibrium, this adds thermal energy. The system shifts the equilibrium in the endothermic direction, thus absorbing some of the added thermal energy, countering the stress. The most challenging of the three types of stress considered in this section is the change in volume. By increasing the volume containing a gas phase reaction at equilibrium, we reduce the partial pressures of all gases present and thus reduce the total pressure. Recall that the response of this reaction to the volume increase was to create more of the reactants at the expense of the products. One consequence of this shift is that more gas molecules are created, and this increases the total pressure in the reaction flask. Thus, the reaction responds to the stress of the volume increase by partially offsetting the pressure decrease with an increase in the number of moles of gas at equilibrium. Le Châtelier's principle is a useful mnemonic for predicting how we might increase or decrease the amount of product at equilibrium by changing the conditions of the reaction. From this principle, we can predict whether the reaction should occur at high temperature or low temperature, and whether it should occur at high pressure or low pressure.
|
|||||||
Home  Physical Chemistry Physical Chemistry  Reaction Equilibrium in the Gas Phase Reaction Equilibrium in the Gas Phase  Changes in Equilibrium and Le Châtelier's Principle Changes in Equilibrium and Le Châtelier's Principle |
|||||||
Last Update: 2011-02-16