| General Chemistry is a free introductory textbook on chemistry. See the editorial for more information.... |

|

Home  Physical Chemistry Physical Chemistry  Reaction Rates Reaction Rates  Temperature Dependence of Reaction Rates Temperature Dependence of Reaction Rates |
|||||||||||||||||||||||






|
|||||||||||||||||||||||
Temperature Dependence of Reaction RatesAuthor: John Hutchinson
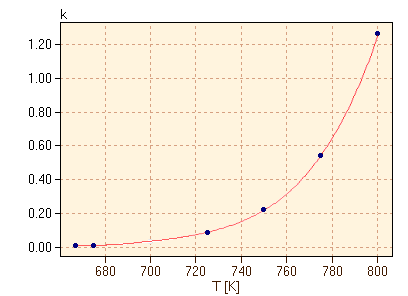
It is a common observation that reactions tend to proceed more rapidly with increasing temperature. Similarly, cooling reactants can have the effect of slowing a reaction to a near halt. How is this change in rate reflected in the rate law equation, e.g. equation 9? One possibility is that there is a slight dependence on temperature of the concentrations, since volumes do vary with temperature. However, this is insufficient to account for the dramatic changes in rate typically observed. Therefore, the temperature dependence of reaction rate is primarily found in the rate constant, k. Consider for example the reaction of hydrogen gas with iodine gas at high temperatures, as given in equation 6. The rate constant of this reaction at each temperature can be found using the method of initial rates, as discussed above, and we find in table 5 that the rate constant increases dramatically as the temperature increases.
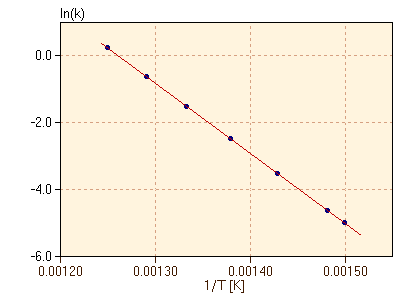
As shown in figure 6, the rate constant appears to increase exponentially with temperature. After a little experimentation with the data, we find in figure 7 that there is a simple linear relationship between ln(k) and 1/T.
From figure 7, we can see that the data in table 4 fit the equation
where a and b are constant for this reaction. It turns out that, for our purposes, all reactions have rate constants which fit equation 17, but with different constants a and b for each reaction. figure 7 is referred to as an Arrhenius plot, after Svante Arrhenius. It is very important to note that the form of equation 17 and the appearance of figure 7 are both the same as the equations and graphs for the temperature dependence of the equilibrium constant for an endothermic reaction. This suggests a model to account for the temperature dependence of the rate constant, based on the energetics of the reaction. In particular, it appears that the reaction rate is related to the amount of energy required for the reaction to occur. We will develop this further in the next section.
|
|||||||||||||||||||||||
Home  Physical Chemistry Physical Chemistry  Reaction Rates Reaction Rates  Temperature Dependence of Reaction Rates Temperature Dependence of Reaction Rates |
|||||||||||||||||||||||
Last Update: 2011-02-20