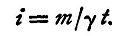| Practical Physics is a free textbook on basic laboratory physics. See the editorial for more information.... |

|

Home  Electricity Electricity  Definition of Electro-Chemical Equivalent Definition of Electro-Chemical Equivalent |
|






|
|
Definition of Electro-Chemical Equivalent
The electro-chemical equivalent of a substance is the number of grammes of the substance deposited by the passage of a unit quantity of electricity through an electrolyte in which the substance occurs as an ion. Thus, if in a time t a current i deposits m grammes of a substance whose electro-chemical equivalent is γ, it follows from the above definition, in conjunction with Faraday's law, that
and hence
If, then, we observe the amount of a substance, of known electro-chemical equivalent, deposited in time t, we can find the current, provided it has remained constant throughout the time t. If a current be allowed to pass between two plates of copper immersed in a solution of sulphate of copper, the sulphate is electrolysed and copper deposited on the kathode. The acid set free by the electrolysis appears at the anode, and combines with the copper. The quantity of copper deposited on the kathode in one second by a unit current has been found to be 0.00328 g. This is the electro-chemical equivalent of copper. The loss of weight of the anode is for various reasons found to be somewhat in excess of this.
|
|
Home  Electricity Electricity  Definition of Electro-Chemical Equivalent Definition of Electro-Chemical Equivalent |
|
Last Update: 2011-03-15