| Learning by Simulations has been developed by Hans Lohninger to support both teachers and students in the process of knowledge transfer and acquisition . Click here for more information. |

Home  Chemistry Chemistry  Atomic Spectra Atomic Spectra |
|||||||||
| See also: Radiation of a Blackbody | |||||||||
Share this Page:






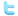
Atomic spectra may be used to quantitatively determine more than 70 chemical elements. In order to be able to detect the atomic spectrum, the atoms or ions have to be separated from one another, i.e. the atoms have to be in a gaseous state. This can be achieved by massively heating the substances to be determined (typically the temperature is several thousand degrees). When the gas heats up, it emits light of various characteristic wavelengths. For hydrogen the resulting spectrum obeys a very simple relation:
where ni and nf are positive non-zero integers with ni > nf and RH is a constant called Rydberg's constant:
RH = 1.097 Apart from chemical analysis atomic spectroscopy is of considerable importance for the investigation of stars, looking for elemental compositions which could enable life in some form. The interest in atomic emission and absorption lines can be traced back to the late 18th and the early 19th Century when Fraunhofer discovered dark lines in the spectrum of the sun light.
If you would like to experiment with spectral lines of selected elements, you can use the program AtomSpec which allows the user to select from several chemical elements. The spectrum may be zoomed to view details of the atomic spectral lines.
|
|||||||||
Last Update: 2006-Jul-04


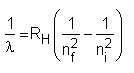
 107 m-1.
107 m-1.