| Learning by Simulations has been developed by Hans Lohninger to support both teachers and students in the process of knowledge transfer and acquisition . Click here for more information. |

Home  Chemistry Chemistry  Acid-Base Titration Acid-Base Titration |
|||||||
| See also: Polyprotic Acids | |||||||
Share this Page:






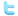
The acid-base titration is an analytical method to determine the concentration of an acid or base in aqueous solutions by neutralizing the acid/base in solution by a basic/acidic reagent. The endpoint of the solution depends on the relative strengths of the used acids and bases. A neutral solution will be obtained only if a strong acid reacts with a strong base, any other combination will shift the endpoint either towards the acidic direction (lower pH values) or more basic solutions (pH values above 7.0). Thus the endpoint of a titration must be indicated by a proper acid-base indicator whose color changes at the pH value of interest.
Many thanks to Ihor Patsay, Ivan Franko National University of Líviv, Ukraine, who contributed the acid-base titration simulation to the Learning by Simulations Web site.
|
|||||||
Last Update: 2008-Feb-27